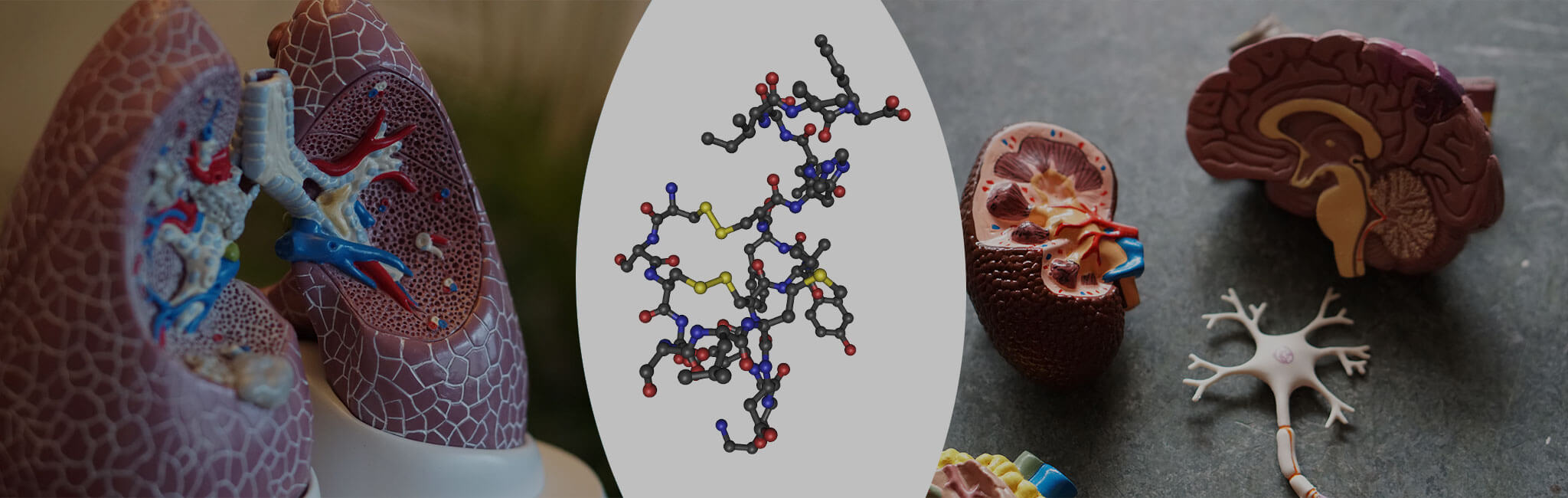
Developing products that are first-in-class with unique mechanism of action and are different from those of existing therapies
Pharmazz, Inc.
Pharmazz, Inc. is an innovative biopharmaceutical company with an approved product, promising drug pipeline and a seasoned management team. It is a Delaware Corporation based in Willowbrook, Illinois, USA focused on discovering, acquiring, developing, and commercializing therapeutics that target critical care medicine. Pharmazz India Private Limited a majority owned subsidiary of Pharmazz, Inc. obtained marketing authorization of Lyfaquin® (INN: Centhaquine) in India.
Pharmazz has licensed exclusive worldwide rights to several molecules indicated for critically ill patients from Midwestern University, Downers Grove, USA. The company has a strong patent position and robust pipeline in different stages of clinical development.

Product Pipeline
| Indication | Pre-Clinical | Phase 1 | Phase 2 | Phase 3 | Market | |
|---|---|---|---|---|---|---|
|
|
||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
|
||||||
| India | U.S. |

Hypovolemic Shock
Centhaquine (Lyfaquin®) is a novel, first-in-class resuscitative agent for the treatment of hypovolemic shock...
Read MoreSeptic
Shock
Septic shock is defined as a life-threatening organ dysfunction caused by a dysregulated host response to ...
Read MoreAcute Kidney Injury
Acute kidney injury (AKI) is a common complication in critically ill patients and is associated with increased...
Read MoreCardiac
Arrest
An experimental study was performed using an established model of cardiac arrest. Cardiopulmonary...
Read MoreCerebral Ischemic Stroke
Stroke is an important cause of serious long-lasting disability. Ischemic stroke caused by arterial occlusion...
Read MoreAlzhieimer's Disease
Alzheimer s disease is the sixth-leading cause of death in the United States. An estimated 5.3 million Americans...
Read MoreAcute Spinal Cord Injury
It is now known that progenitor stem cells are present in the spinal cord.Exposure of cultured neural progenitor cells to hypoxia also...
Read More
Management
The management team is well balanced with significant drug development and clinical expertise, and commercialization experience. In addition to the management and development team in the US, the company has over 80 employees through its subsidiary in India.
More Details
Dr. Gulati is leading clinical development and commercialization of first-in-class drug products in critical care medicine. He led the discovery, development and launch of Lyfaquin for hypovolemic shock & Tyvalzi for cerebral stroke.

Dr. Neil Marwah is a seasoned, entrepreneurial healthcare executive with more than 30 years of experience. His experience includes private practice, ancillary healthcare services, large provider organizations, private equity, and senior management at Global 500 enterprise.

Dr. McDonnell received his undergraduate degree from Georgetown University and his MD in 1985 from Northwestern University. Dr. McDonnell has also received postgraduate training in Emergency and Internal Medicine at Northwestern University.

Mr. Costello brings about 20 years of accounting, budgeting, audit preparation, reporting and financial modeling experience to Pharmazz. He served as Controller for Julian Toft and Downey, Inc. and as Vice President in Transwestern Commercial Real Estate's

Dr. Gulati has more than 38 years of experience and knowledge in starting and running medium sized organizations. He is the founding member of Pharmazz India Private Limited and is managing and operating the company since its inception.
Press Releases
Media Coverage
CHI Memorial First In Region To Enroll Patient In Groundbreaking Stroke Clinical Trial
CHI Memorial is proud to announce it is the first hospital in the world to enroll a patient in a Phase 3 clinical trial evaluating a promising new treatment for ischemic stroke. This groundbreaking...
Pharmazz, Inc. showcased the groundbreaking potential of its lead drug, Sovateltide
Pharmazz, Inc., a biopharmaceutical company addressing the critical care market, showcased the groundbreaking potential of its lead drug...
Pharmazz Chairman and CEO Dr. Anil Gulati’s IPC-IPSCON 2024 session sheds
By APN News On Nov 30, 2024 -- Pharmazz, Inc., a late-stage biopharmaceutical company addressing the critical care market, showcased the groundbreaking...